Abstract
Introduction
Older patients with severe aplastic anemia (SAA) needing hematopoietic cell transplantation (HCT) typically require utilization of reduced intensity conditioning (RIC) regimens; however, use of RIC regimens in this population is limited by graft failure and transplant-related mortality due to toxicity and infections, particularly reactivation of Epstein-Barr virus (EBV). Novel RIC regimens with improved efficacy and decreased toxicity and risk of infectious complications are urgently needed to improve post-HCT outcomes for SAA patients.
Patients and Methods
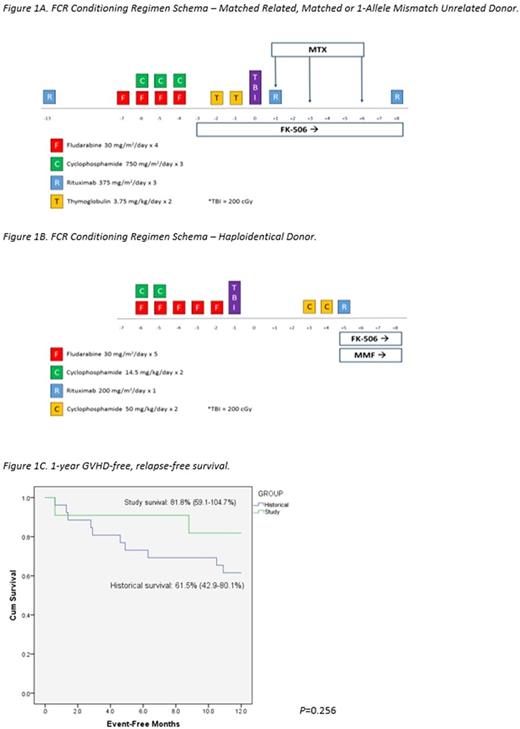
Utilizing institutional registries, we identified all SAA patients who underwent first allogeneic HCT using an FCR regimen (Figures 1A and 1B) between August 2014 and May 2017 at our institution for inclusion in this analysis. Post-transplant immune suppression comprised of tacrolimus and either methotrexate or mycophenolate mofetil. The Institutional Review Board approved this analysis and a waiver of informed consent was obtained. Medical records of all patients were reviewed by participating investigators. Assessment of graft-versus-host disease (GVHD) was performed following standard grading practice. An analysis of 1-year GVHD-free, relapse-free survival (GRFS) was conducted and compared to an historical cohort of all SAA patients transplanted with a non-FCR regimen at our institution since January 1999 (n=27), with differences between the curves determined using log-rank tests.
Results
11 SAA patients underwent HCT from August 1, 2014 to May 5, 2017, the majority of which were male with a median age of 41 years (range: 19-64). Most patients received one line of treatment prior to HCT (CSA+ATG=6, CSA=1, steroids=1) and received an HCT within a median of 205 days after diagnosis (range: 70-2090). All patients received transplants from matched or haploidentical donors (matched related=4, matched unrelated=5, haploidentical=2) using a peripheral blood stem cell source, except 1 patient who received a bone marrow graft. At median follow-up of 302 (range: 49-911) days post-transplant, all patients were alive and in remission with 100% donor peripheral blood chimerism with bone marrow biopsies documenting normal trilineage hematopoiesis. Average time to neutrophil and platelet engraftment was 15.4 days and 16 days, respectively. The median number of inpatient hospital days required in the first 100 days post-transplant was 11 days (range: 3-29). By day 100 post-transplant, 54.5% of patients developed some degree of acute GVHD (grade I=1, grade II=3, grade III=2). However, by date of last follow-up, all evaluable patients (10/11) had not developed chronic GVHD. Only 2 patients experienced a viral reactivation (1=cytomegalovirus (CMV), 1=CMV+EBV) by date of last follow-up post-transplant. The case of EBV reactivation did not require treatment and no patients developed post-transplant lymphoproliferative disorder. The unadjusted Kaplan-Meier estimate of 1-year GRFS was 81.8% (95% CI: 59.1-104.7%) in the study group versus 61.5% (95% CI: 42.9-80.1%) in the historical group (P=0.256) (Figure 1C) with death accounting for the greatest proportion of GRFS events in the historical population and grade III acute GVHD accounting for the two GRFS events in the study cohort.
Conclusions
The positive results from this study demonstrating low toxicity, GVHD, and EBV reactivation rates with no graft failure or relapse potentially justify the use of these novel outpatient non-myeloablative conditioning regimens in SAA patients of any age receiving transplantation from any donor source. Further prospective investigation in a larger population is warranted.
Gatwood: Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Byrne: Pfizer: Membership on an entity's Board of Directors or advisory committees; Concert Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding. Jagasia: Therakos: Consultancy, Research Funding; Mallinckrodt: Consultancy; Janssen: Consultancy, Research Funding. Savani: Jazz Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal